Comaiba for Clinical Research
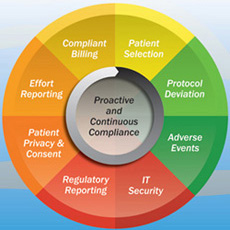
Compliance management is an ongoing challenge for the global clinical research industry. The need to meet regulations from the Centers for Medicare and Medicaid Services (CMS), the Office of the Inspector General (OIG), the US Food and Drug Administration (FDA), local regulatory bodies in individual countries and multiple Institutional or Ethical Review Boards (IRBs) is often met through loosely structured processes, a mix of paper and multiple electronic systems working in parts, and teams of consultants. The approach is often case-based for each trial, and is always expensive and reactive. It is also not particularly effective at preventing or identifying deviations, adverse events, billing errors, fraud, abuse and waste. These problems occur at the granular trial level, and at the systemic or institutional level.
A fragmented approach to managing risk makes it impossible to stay on top of enterprise-wide risks. Critical risks are likely to cut across multiple domains that single-point, focused solutions can neither predict nor capture risks or deviations. For example, a single clinical trial study can have patient safety risk due to adverse events, privacy and other risks related to informed consent, regulatory and execution risk from non-conformance with FDA regulations and financial risks due to the potential for double-billing.
Comaiba for clinical research provides a comprehensive compliance solution to improve patient safety and reduce overall costs of conducting clinical trials. Institutions can significantly reduce unnecessary screening, inappropriate enrollment and incorrect execution. For all stages of a clinical trial, Comaiba sets up and execute rules that span multiple systems such as lab, billing and clinical trial management software.
Comaiba provides the capability to track key measures such as patient eligibility, access violations and billing violations over time. Continuous monitoring of clinical assessments performed during the study can also be setup to maintain aggressive vigilance for patient safety. Any violations are captured in real time as and when they happen, audited and reported via dashboard alerts to trial investigators and to IRBs (Internal Review Board).